Pipeline
A new approach for correctors therapies
Protein misfolding and mistrafficking causes devastating diseases affecting hundreds of thousands of patients globally. Small molecule correctors are a proven therapeutic approach for such diseases, but have been mostly confined to the small subset of misfolding diseases with a few dominant mutations. The Octant Navigator platform uses Generative Biology and Generative Chemistry to pursue multi-mutant correctors, offering new hope to treat many of these diseases.
Our Pipeline
See how we're harnessing our platform to build therapies that deliver relief to patients.
OCT-980: A New Clinical Approach for Retinitis Pigmentosa
Retinitis Pigmentosa (RP) is a group of inherited retinal disorders that cause progressive vision loss and can lead to blindness. Rhodopsin-associated autosomal dominant RP (RHO-adRP) is among the most severe and prevalent forms, yet no treatment options exist for the thousands of individuals affected by this disease.
OCT-980 is a first-in-disease oral small molecule designed to correct protein misfolding for rhodopsin gene mutations, the root cause of RHO-adRP. By restoring protein function, OCT-980 aims to improve low-light vision and halt disease progression. Preclinical studies show strong efficacy and a favorable safety profile, supporting advancement into clinical trials in early 2026.
At Octant, we're redefining what's possible in precision medicine by targeting the root causes of genetic diseases and bringing transformative therapies from discovery to the clinic.

Navigating Retinitis Pigmentosa
Drug discovery in engineered cell systems
Octant is using the Navigator to design small molecule correctors that mitigate toxic accumulation of misfolded rhodopsin in the retina. We’ve engineered human cells to read out on trafficking and biophysical properties of RHO mutations, generating millions of data points from each screen that guide drug design.
Multiplexing to increase patient coverage
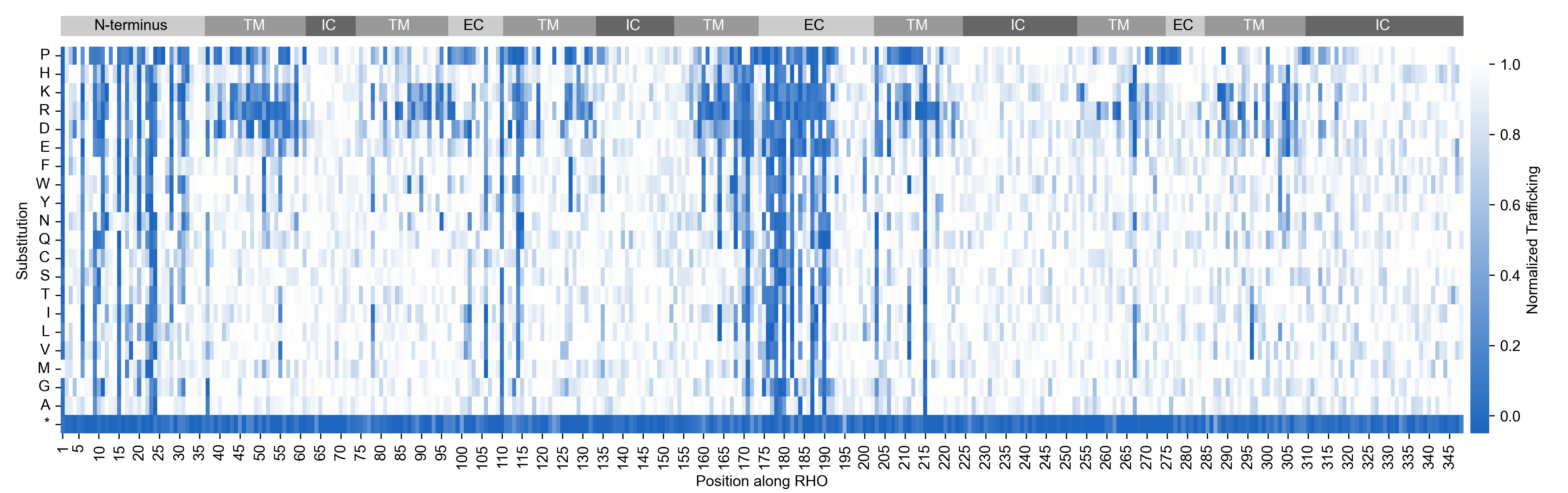
While most rare disease programs initially target a single mutation, our programs apply multi-mutant approaches that maximize the addressable patient population from the start. We engineer different cell lines for different mutations, each with an RNA barcode that identifies the variant. Using deep mutational scanning (DMS), Octant measured the efficacy and potency of OCNT-980 by building uniquely barcoded human cell lines for every possible single amino acid variant of RHO, probing the cell lines together under carefully controlled conditions.
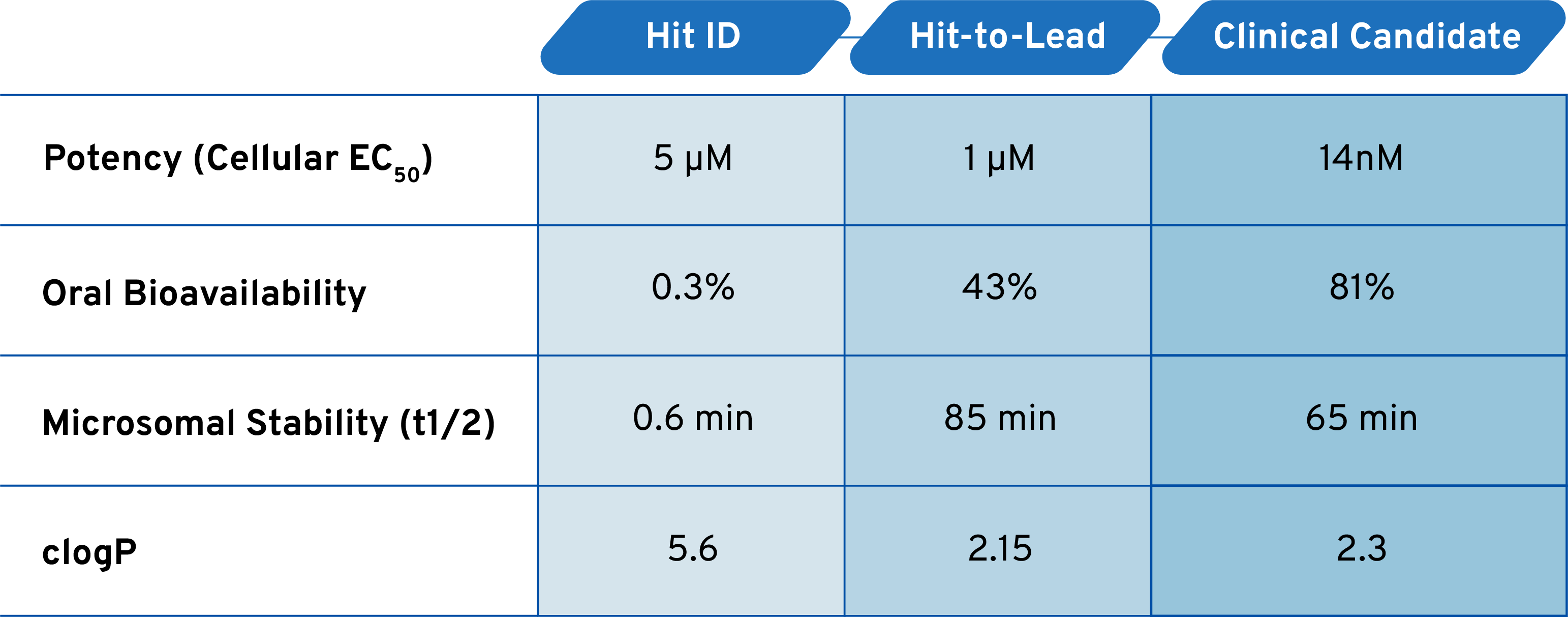
Generative Chemistry Improves Potency and Drug-Like Properties
Nano-scale chemistry enables us to build thousands of analog molecules at a time and optimize them in learning loops. In the RHO-adRP program we iteratively built and screened 250,000+ molecules direct-to-biology, yielding thousands of structure-activity insights. We transformed early crude compounds with low potency and poor drug-like properties into potent, stable, orally available correctors that cross the blood-retinal barrier and engage the target in the retina to produce in vivo efficacy.
Developing correctors for misfolding diseases requires a new approach to drug discovery
The correct Cellular Context
Predicting the structure of a protein is hard. Modeling the cellular journey of a misfolded protein, how the misfolding affects the protein’s function, and the cellular response to that stress, is even harder. We’ve developed a Generative Biology platform for studying protein misfolding in human cells, and to conduct high-throughput screening in that environment.
Multiplexed Read Outs
Engineering small molecule correctors requires developing a drug against multiple mutations from the start, while also optimizing the molecules for drug-like qualities. Octant’s Generative Biology platform enables the multiplexed measurement of the many biological phenomena associated with protein folding and trafficking across hundreds of mutations and conditions. This enables us to fine-tune our compounds to make effective new therapies for the most patients.
Direct-to-biology HT-SAR
Correcting misfolded proteins is a molecular dance in a crowded cellular environment. It’s difficult to progress correctors of protein misfolding because traditional methods that rely on crystal structures and biochemical binding are often not amenable to unfolded proteins. Instead, the Octant Navigator weekly synthesizes and screens thousands of chemical analogs, iterating on a molecule’s efficacy and drug-like qualities. This combines the rationality of SAR with the serendipity of phenotypic screening to enable machine learning and accelerate the empirical discovery of new corrector therapies.

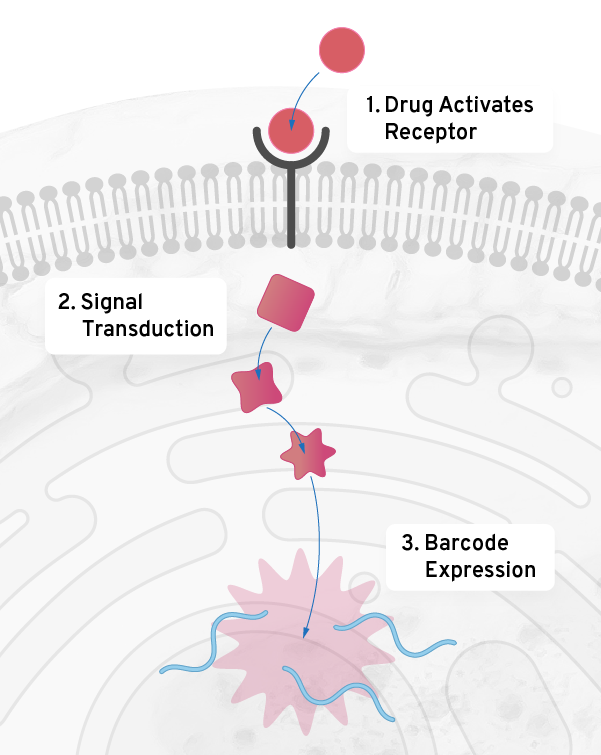
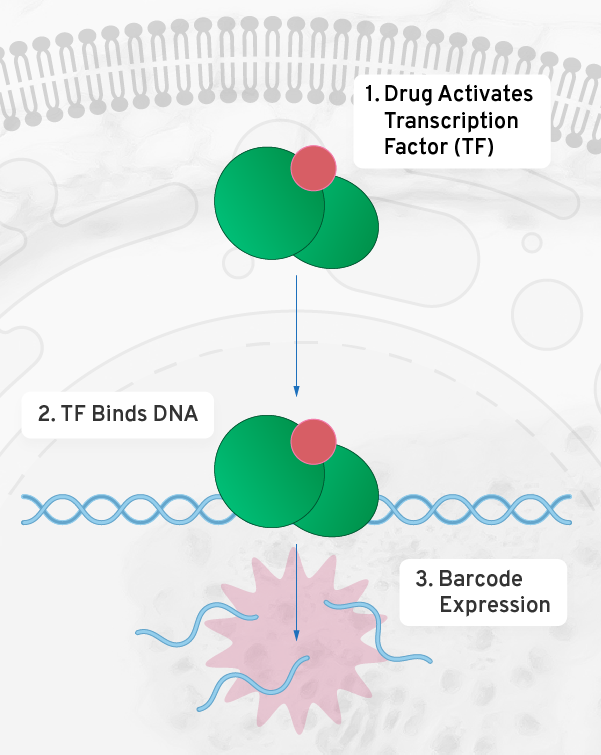
Other applications of mechanism based screening
Cellular mechanisms are fundamental to many complex problems in drug discovery. Using our genetic barcoding technology we model and screen against mechanisms ranging from transcription to the signaling pathways associated with GPCRs and kinases. The Octant Navigator is well-suited to high value targets from CNS to metabolism to immunology.
Contact us
If you want to transform drug discovery with us, we'd love to hear from you.
ahoy@octant.bio

